
In contrast, Adstiladrin uses a “novel” Ad5 vector, says Patrick Gorman, a spokesman for Ferring, adding that therapeutic uses of adenovirus vectors have been increasingly well characterised in recent years. It should also be stressed that the Gelsinger case involved an extremely high systemic dose, and an early-generation adenovirus. One possibility might be that the bladder, into which Adstiladrin is administered via a urinary catheter, is more immune privileged than thought. “The subtleties on how Ferring is likely able to repeat administer here are important.” “It’s clearly possible to repeat administer adenovirus,” she says. However, she hastens to cite the example of Astrazeneca’s Covid vaccine Vaxzevria, which also uses an adenoviral vector and is dosed two or three times. “Fundamentals of virology and vaccinology tell us that repeat exposure to or any virus should absolutely lead to the development of a massive neutralising antibody response against the various viral antigens,” Professor Nicole Paulk, who specialises in AAV-based gene therapies at University of California San Francisco, tells Vantage. Those with a long enough memory will recall the death of Jesse Gelsinger, attributed to his development of a severe immune reaction against a gene therapy. Normally, repeat dosing of an adenoviral vector would result in dangerous immunogenicity. Additionally, the therapy is designed to be given every three months rather than as a once-and-done procedure. Potential problems extend beyond the complexity of manufacturing a gene therapy, and include Adstiladrin’s use of a non-replicating adenovirus serotype 5 (Ad5) vector. Rather, it merely encodes the gene for human interferon alfa-2b, and it is this cytokine, once produced locally, that exerts antitumour activity. Asked by Evaluate Vantage, Ferring would not comment on its commercial plans.īut why is Adstiladrin controversial? The product is not what many people would consider a typical gene therapy in that it does not seek to alter or correct a disease’s underlying genetic defect. The company does have a US commercial presence, but says it still needs to expand manufacturing capacity to be able to produce vector for oncology at commercial scale.

One possibility is that Ferring needs a US licensee, having recently lost Blackstone as its joint-venture partner. Surprisingly, Ferring has still not launched it, and does not expect to until the second half of 2023. Adstiladrin was originated by Merck & Co, attracted $400m of financing from Blackstone, saw off one chief executive and survived an FDA rejection. The product’s development history offers a lesson in tenacity. Still, Adstiladrin’s long-term benefits remain elusive, and a cumbersome mechanism and use of an adenovirus vector raise questions about its viability. The product’s tortuous journey is the result of a renewed industry effort to tackle non-muscle invasive bladder cancer, an earlier setting than the metastatic disease that has seen several drugs approved recently.
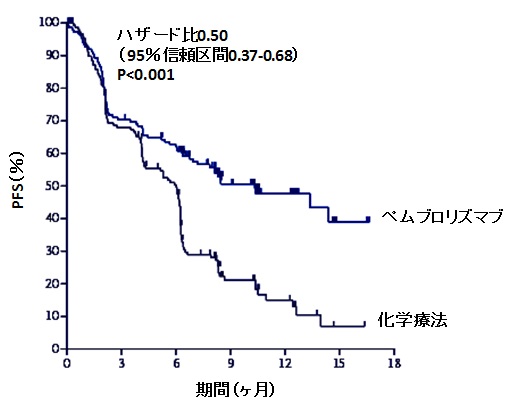
The December US approval of Ferring’s cancer gene therapy Adstiladrin probably passed unnoticed by many biotech investors it occurred during the Christmas lull, and the beneficiary was a private European company best known for reproductive health and the microbiome.


 0 kommentar(er)
0 kommentar(er)
